What Determines the Loss or Gain of Electrons?
Based on the position of elements on the Periodic Table, alkali and alkaline metals in most cases form cations. On the other hand, the halogens and many other non-metals form anions. The alkali metals lose one electron, alkaline metals lose two electrons, and the aluminum and other group three elements lose three electrons. On the contrary, halogens gain one electron; the group 6A elements gain two electrons whereas the group 5A elements gain three electrons. Either one or several atoms and molecules may form an anion. If the anion is formed by one atom, it is called a monoatomic anion. However, when it is formed by two or more atoms or molecules, then it is a polyatomic anion. The loss of one, two, or three electrons lead to the formation of monovalent, divalent, and trivalent cations respectively.
Differences Between a Cation & an Anion
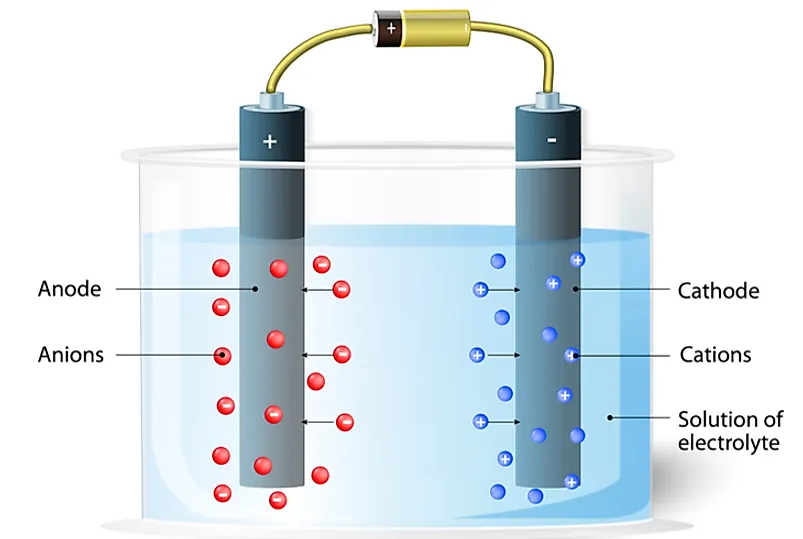
The first difference is that anions possess negative electrical charges while the cations have positive electrical charges. Secondly, during electrolysis, anions move to the positive electrode which is referred to as the anode. However, the cations migrate to the negative electrode called cathode. Another difference between the two ions is that anions have more electrons than protons while the cations have more protons than electrons. Then there’s the difference that comes due to the etymology of the words. Anion comes from the Greek word “ano” which means “up.” On the contrary, the cation derives its meaning from a Greek word “kata” which means “down.” Finally, anions are non-metals while the cations are metals.
Application of The Concept of Anions & Cations
Ions conduct electricity through liquids or solutions. Hence the anions and cations are responsible for the phenomenon of the flow of electric current in a dry cell. The current will always flow towards the direction of the positive charge. On the other hand, it moves to the opposite direction of the flow of the negative charge carriers. In a dry cell, electricity flows out of the cathode and flows into the anode. The cathode is negatively charged, attracting positive charges. On the other hand, the anode is positively charged drawing only the negative charge.


 Users Today : 334
Users Today : 334 Total views : 468077
Total views : 468077